HIV Disease BackgroundHuman
immunodeficiency virus (HIV) disease was first described in 1981 among 2
groups—one in San Francisco and the other in New York City. Numerous
young homosexual men presented with opportunistic infections that, at
the time, were typically associated with severe immune deficiency due to
Pneumocystis pneumonia (PCP) or aggressive Kaposi sarcoma.
[1] The HIV virus itself was not identified for another 2 years
[2] ;
during that time, various other causes were considered, including
lifestyle factors, chronic drug abuse, and other infectious agents.
[3] The
HIV epidemic spread rapidly and silently in the absence of testing.
However, clear clinical implications arose before society became aware
of the disease; for example, prior to the recognition of HIV, only one
case of
Pneumocystis pneumonia not clearly associated with
immune suppression was diagnosed in the United States between January
1976 and June 1980. In 1981 alone, 42 similar diagnoses were made, and,
by December 1994, 127,626 cases of
Pneumocystis pneumonia with
HIV infection as the only identified cause of immune suppression had
been reported to the Centers for Disease Control and Prevention (CDC).
Also, Kaposi sarcoma is up to 30,000 times more likely to develop in
persons with HIV infection than in immunocompetent persons. HIV
is a blood-borne, sexually transmissible virus. The virus is typically
transmitted via sexual intercourse, shared intravenous drug
paraphernalia, and mother-to-child transmission (MTCT), which can occur
during the birth process or during breastfeeding. The most common route
of infection varies from country to country and even among cities,
reflecting the population in whom HIV was introduced initially and local
practices. Co-infection with other viruses that share similar routes of
transmission, such as hepatitis B, hepatitis C, and human herpes virus 8
(HHV8; also known as Kaposi sarcoma herpes virus [KSHV]), is common. Two
distinct species of HIV (HIV-1 and HIV-2) have been identified, and
each is composed of multiple subtypes, or clades. All clades of HIV-1
tend to cause similar disease, but the global distribution of the clades
differs. This may have implications on any future vaccine, as the B
clade, which is predominant in the developed world (where the large
pharmaceutical companies are located), is rarely found in the developing
countries that are more severely affected by the disease. HIV-1 probably originated from one or more cross-species transfers from chimpanzees in central Africa.
[4] HIV-2 is closely related to viruses that infect sooty mangabeys in western Africa.
[5] Genetically,
HIV-1 and HIV-2 are superficially similar, but each contains unique
genes and its own distinct replication process. HIV-2 carries a
slightly lower risk of transmission, and HIV-2 infection tends to
progress more slowly to acquired immune deficiency syndrome (AIDS). This
may be due to a less-aggressive infection rather than a specific
property of the virus itself. Persons infected with HIV-2 tend to have a
lower viral load than people with HIV-1
[6, 7] , and a greater viral load is associated with more rapid progression to AIDS in HIV-1 infections.
[8, 9] Because
HIV-2 is rare in the developed world, most of the research and vaccine
and drug development has been (perhaps unfairly) focused on HIV-1 (see
Deterrence/Prevention). See the image below.
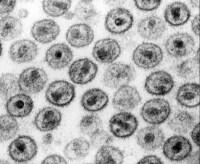
Electron microscopy of human immunodeficiency virus (HIV)–1 virions. Courtesy of CDC/Dr. Edwin P. Ewing, Jr. A
considerable amount of stigma has been attached to HIV infection,
mostly because of the virus's association with sexual acquisition and
the inference of sexual promiscuity. Consequences of this stigma have
included discrimination and reluctance to be tested for HIV infection.
The stigma of HIV infection is also associated with a fear of acquiring a
rapidly fatal infection from relatively casual contact. Such attitudes
are inappropriate because HIV is poorly transmissible without sexual
contact or blood contact. In addition, the expected survival is long in
patients with HIV infection who are receiving treatment. HIV is not
transmitted during casual contact and is readily inactivated by simple
detergents. Much of the concern regarding HIV infection is due to the
incurability of the infection and the relentless immune decline and
eventual premature death in the vast majority of infected people. The
spread of HIV was retrospectively shown to follow the trucking routes
across Africa from logging camps, and the bush-meat trade combined with
aggressive logging and improved transportation in the mid-20th century
may have allowed what was likely occasional cross-species transmission
events to propagate across the country and, eventually, the globe.
[10] Since
the discovery of HIV and its link to acquired immune deficiency
syndrome (AIDS), great strides have been made in understanding its
biology and in developing effective treatments. The difficulty in
dealing with HIV on a global scale is largely due to the fact that HIV
infection is far more common in resource-poor countries. In the
developed world, antiretroviral therapy has greatly improved prognosis
and increased survival rates. Public education programs have raised
awareness such that testing and prevention of infection are more common.
Both of these approaches are difficult in countries with undereducated
or underfunded populations. Political denial and inaction have
also likely caused considerable damage. Several governments in countries
with high HIV infection rates were slow to admit that they had an HIV
epidemic, and at least one (South Africa) initially rejected that AIDS
was even a problem, then that the disease was caused by HIV infection,
and, most recently, that antiretroviral therapy was effective in
treating HIV infection and preventing MTCT. Changes have now occurred
but have been slow and have had an unknown cost. For supplementary information, see the eMedicine articles Early Symptomatic HIV Infection and HIV Infection and AIDS.
Pathophysiology
Virology of HIVHIV-1 and HIV-2 are retroviruses in the Retroviridae family,
Lentivirus genus. They are enveloped, diploid, single-stranded, positive-sense RNA
viruses with a DNA intermediate, which is an integrated viral genome (a
provirus) that persists within the host-cell DNA. There is no fixed
site of integration, but the virus tends to integrate in areas of active
transcription, probably because these areas have more open chromatin
and more easily accessible DNA.
[11, 12] This
greatly complicates eradication of the virus by the host, as latent
proviral genomes can persist without being detected by the immune system
and cannot be targeted by antivirals. See the image below.
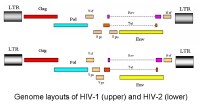
Genome layout of human immunodeficiency virus (HIV)–1 and HIV-2. HIV contains the 3 species-defining retroviral genes—
gag (group-specific antigen; the inner structural proteins),
pol (polymerase; also contains integrase and protease—the viral enzymes—and
is produced as a C-terminal extension of the Gag protein), and
env (envelope; the outer structural proteins responsible for cell-type specificity). HIV-1 has 6 additional accessory genes—
tat, rev, nef, vif, vpu, and
vpr. HIV-2 does not have
vpu but instead has the unique gene
vpx. The only other virus known to contain the
vpu gene is simian immunodeficiency virus in chimpanzees (SIV
cpz), which is the simian equivalent of HIV.
[4] Interestingly, chimpanzees with active HIV-1 infection are resistant to disease.
[13] The accessory proteins of HIV-1 and HIV-2 are involved in viral replication and may play a role in the disease process.
[14, 15] The
outer part of the genome consists of long terminal repeats (LTRs) that
contain sequences necessary for gene transcription and splicing, viral
packaging of genomic RNA, and dimerization sequences to ensure that 2
RNA genomes are packaged. The dimerization, packaging, and
gene-transcription processes are intimately linked; disruption in one
process often subsequently affects another. The LTRs exist only in the
proviral DNA genome; the viral RNA genome contains only part of each
LTR, and the complete LTRs are re-created during the
reverse-transcription process prior to integration into the host DNA.
The biologic basis for AIDSThe
specific details of the disease process that leads to AIDS are not
fully understood despite considerable progress in the virology of HIV
and the immunology of the human host, much of which has been driven by
the urge to better understand AIDS.
[16, 17, 18] There is a specific decline in the CD4
+ helper T cells, resulting in inversion of the normal CD4/CD8 T-cell ratio and dysregulation of B-cell antibody production.
[19, 20] Immune
responses to certain antigens begin to decline, and the host fails to
adequately respond to opportunistic infections and normally harmless
commensal organisms. Because the defect preferentially affects cellular
immunity, the infections tend to be nonbacterial (fungal, viral). The
pattern of opportunistic infections in a geographic region reflects the
pathogens that are common in that area. For example, persons with AIDS
in the United States tend to present with commensal organisms such as
Pneumocystis and
Candida species, homosexual men are more likely to develop Kaposi sarcoma because of co-infection with HHV8, and tuberculosis is common in developing countries. Recent work has shown the importance of gut-associated lymphoid tissue (GALT) in HIV replication.
[21] Although
the portal of entry for HIV infection is typically through direct blood
inoculation or exposure of the virus to genital mucosal surfaces, the
GI tract contains a large amount of lymphoid tissue, making this an
ideal site for HIV replication. GALT has been shown to be a site
of early viral seeding and establishment of the proviral reservoir. This
reservoir contributes to the difficulty of controlling the infection,
and efforts to reduce the levels of HIV provirus through sustained
antiretroviral therapy (alone or in combination with interleukin-2
activation of resting HIV-infected T cells) have consistently failed.
[22] A feature of HIV replication in GALT is that it is compartmentalized, even among different areas of the gut.
[23] Measurements of CD4
+ T cells in GALT show relatively less reconstitution with antiretroviral therapy than that observed in peripheral blood.
[24, 25] At least one report has suggested that early treatment may result in better GALT CD4 T-cell recovery
[25] ,
but clinical data generally argue against early initiation of therapy,
which has not been shown to improve long-term survival. In addition, HIV
replication can be detected even in patients with supposedly suppressed
replication, as judged by plasma viral load measurements. CD8
+ killer T-cell responses to HIV occur in GALT and do not decline with antiviral therapy as much as peripheral measurements do.
[26] These findings underscore the limitations of peripheral measurements in what is really a central viral replication. One
theory for the discrepancy between GALT and blood measurements is that
ongoing viral replication in the lymphoid tissue, and the resulting
immune activation, may actually hamper efficient CD4
+ T-cell replenishment.
[27] Studies
of T-cell–replication kinetics have revealed that untreated HIV
infection is characterized by rapid T-cell turnover but a defect in
T-cell replication from the thymus.
[28, 29, 30] These changes can be reversed with effective long-term antiviral therapy,
[31, 32] suggesting
that they are due to a direct effect of the virus or are a feature of
the immune response against HIV. It is known that normal cell cycling is
necessary to produce a normal cytokine profile
[33] and that HIV causes cell-cycle arrest,
[34] but whether this is the exact mechanism is unresolved. Several
of the HIV proteins directly affect T-cell function, either by
disrupting cell cycling or down-regulating the CD4 molecule. The loss of
T cells is clearly a primary issue, as the T-cell repertoire narrows in
terms of which antigens the immune system will recognize and respond
to. Antiviral therapy is able to reverse these changes,
[35] but
the degree of reversal is decreased if therapy is initiated very late
in the infection and is further decreased when therapy is initiated when
CD4 T-cell counts are 200/μL and below. Direct cytotoxic effects of
viral replication are likely not the primary cause of CD4 T-cell loss; a
significant bystander effect
[36] is
likely secondary to T-cell apoptosis as part of immune hyperactivation
in response to the chronic infection. Infected cells may also be
affected by the immune attack. One interesting issue is that the
co-receptor usage of the virus strains tends to change over time. The
initial infection nearly always involves a strain that uses the
chemokine receptor 5 (CCR5) co-receptor found on macrophages and
dendritic cells. People who are homozygous for deletions in the
CCR5 gene tend to be resistant to infection and may have some protection against progression.
[37, 38] Over
time, the receptor usage shifts to chemokine-related receptor (CXCR4)
and other related receptors found on CD4 T cells. These virus strains
are more likely to cause cell fusion (syncytia formation). This trend is
far from absolute but does correlate in many people with disease
progression.
[39] A
single case report detailed a possible cure resulting from stem-cell
transplantation from a CCR5-delta32 homozygous donor (performed to treat
acute myelocytic leukemia). Although this important finding is unlikely
to impact routine management of HIV infection, it does suggest that
reconstitution of a host immune system with a population of mutant cells
is a possible avenue of research to explore.
[40] Regardless
of the cause for the disruption, a loss of thymic replacements in the
face of an induced state of immune activation and T-cell loss seems to
be a key component of the mechanism by which HIV narrows the T-cell
repertoire and progresses to AIDS.
[41, 42, 43] Visible
effects of HIV infection come in the form of disrupted lymph-node
architecture. This disruption is temporal, and, at one point, lymph-node
biopsy was considered as a form of staging the disease.
[44, 45] The
disruption of the follicular dendritic network in the lymph nodes and
subsequent failure of normal antigen presentation are likely
contributors to the disease process. HIV replicates in activated T cells
(its promotor is a nuclear factor kappa B [NF-kappa-B]–binding region,
the same protein that promotes other proteins in activated T cells and
macrophages), and activated T cells migrate to the lymph nodes. As such,
much of the viral replication occurs outside of the peripheral blood,
even though serum viral load is still a useful surrogate marker of viral
replication.As mentioned above, with regards to GALT, HIV
infection may be compartmentalized; specifically, areas of
immune-privilege may occur such as in the testes and central nervous
system where not only will there be differences in HIV pseudospecies but
also different degrees of antiretroviral drug penetration. There is
evidence that even with good peripheral control of HIV, it may still be
detectable in the CSF of some infected patients.
[46] Phases of HIV InfectionClinical
HIV infection undergoes 3 distinct phases—acute seroconversion,
asymptomatic infection, and AIDS. Each is discussed below.
Acute seroconversionDuring this phase, the infection is established, and a proviral reservoir is created.
[47, 48] This
reservoir consists of persistently infected cells, typically
macrophages, and appears to steadily release virus. Some of the viral
release replenishes the reservoir, and some goes on to produce more
active infection. The proviral reservoir, as measured by DNA polymerase
chain reaction (PCR), seems to be incredibly stable. Although it does
decline with aggressive antiviral therapy, the half-life is such that
eradication is not a viable expectation. The size of the proviral
reservoir correlates to the steady-state viral load and is inversely
correlated to the anti-HIV CD8 T-cell responses. Aggressive early
treatment of acute infection may lower the proviral load, but,
generally, treatment in newly infected (but postseroconversion) patients
yields no long-term benefit. At this point, the viral load is
typically very high, and the CD4 T-cell count drops precipitously. With
the appearance of anti-HIV antibodies and CD8 T-cell responses, the
viral load drops to a steady state and the CD4 T-cell count returns to
levels within the reference range, although slightly lower than before
infection. Seroconversion may take a few weeks, up to several
months. Symptoms during this time may include fever, flulike illness,
lymphadenopathy, and rash and develop in approximately half of all
people infected with HIV.
Asymptomatic HIV infectionAt
this stage in the infection, persons infected with HIV exhibit few or
no signs or symptoms for a few years to a decade or more. Viral
replication is clearly ongoing during this time,
[49] and
the immune response against the virus is effective and vigorous. In
some patients, persistent generalized lymphadenopathy is an outward sign
of infection. During this time, the viral load, if untreated, tends to
persist at a relatively steady state, but the CD4 T-cell count steadily
declines. This rate of decline is related to, but not easily predicted
by, the steady-state viral load. No firm evidence has shown that
the initiation of therapy early in the asymptomatic period is effective,
although very late initiation is known to result in a less effective
response to therapy and a lower level of immune reconstitution.
AIDSWhen
the immune system is damaged enough that significant opportunistic
infections begin to develop, the person is considered to have AIDS. For
surveillance purposes in the United States, a CD4 T-cell count less than
200/μL is also used as a measure to diagnose AIDS, although some
opportunistic infections develop when CD4 T-cell counts are higher than
200/μL, and some people with CD4 counts under 200/μL may remain
relatively healthy. Many opportunistic infections and conditions
are used to mark when HIV infection has progressed to AIDS. The general
frequency of these infections and conditions vary from rare to common
but are uncommon or mild in immunocompetent persons. When one of these
is unusually severe or frequent in a person infected with HIV and no
other causes for immune suppression can be found, AIDS can be diagnosed.
[50]
Opportunistic infections and conditionsEven
after starting therapy and with effective suppression of viral load,
patients with persistently low CD4 counts remain at high risk for
opportunistic infections. In general, all patients remain at a
relatively high risk for opportunistic infections and other AIDS-related
events for the first 6 months of antiretroviral therapy.
[51] Opportunistic infections and conditions include the following (
* added in the 1993 AIDS surveillance case definition):
- Candidiasis of bronchi, trachea, or lungs
- Candidiasis, esophageal
- Cervical cancer, invasive*
- Coccidioidomycosis, disseminated or extrapulmonary
- Cryptococcosis, extrapulmonary
- Cryptosporidiosis, chronic intestinal (duration >1 mo)
- Cytomegalovirus disease (other than liver, spleen, or nodes)
- Cytomegalovirus retinitis (with vision loss)
- Encephalopathy, HIV-related
- Herpes simplex - Chronic ulcer or ulcers (duration >1 mo) or bronchitis, pneumonitis, or esophagitis
- Histoplasmosis, disseminated or extrapulmonary
- Isosporiasis, chronic intestinal (duration >1 mo)
- Kaposi sarcoma
- Lymphoma, Burkitt (or equivalent term)
- Lymphoma, immunoblastic (or equivalent term)
- Lymphoma, primary, of the brain
- Mycobacterium avium complex or Mycobacterium kansasii infection, disseminated or extrapulmonary
- Mycobacterium tuberculosis infection, any site (pulmonary* or extrapulmonary)
- Mycobacterium infection with other species or unidentified species, disseminated or extrapulmonary
- Pneumocystis pneumonia
- Pneumonia, recurrent*
- Progressive multifocal leukoencephalopathy
- Salmonella septicemia, recurrent
- Toxoplasmosis of the brain
- Wasting syndrome due to HIV infection
- See the image below
- .

- Timeline
of CD4 T-cell and viral-load changes over time in untreated human
immunodeficiency virus (HIV) infection. From Wikipedia, based on an
original from Pantaleo et al (1993).
- Epidemiology
Frequency
United States
The
most recent frequency data concerning HIV infection in the United
States are from 2006. According to data from states that have
confidential name-based reporting, the national-average incidence of HIV
infection is 18.5 per 100,000 population. The incidence rate of late
HIV disease (AIDS) is 12.3 per 100,000 population. With improved
estimation methods, the number of new HIV infections in 2006 has been
estimated at 56,300. Approximately 1 million persons have been diagnosed
with AIDS since 1981, and more than 500,000 people have died with AIDS
(although reporting limitations mean that not every "death with AIDS" is
directly attributable to AIDS itself). Approximately 1.1 million people
currently have HIV infection in the United States. US rates vary by state. See the latest Centers for Disease Control (CDC) surveillance report for full details (maps 1 and 2).The
overall figures may give a false impression that the HIV epidemic is
relatively homogenous. In fact, the HIV epidemic is best viewed as
numerous separate epidemics among distinct risk groups, although the
various epidemics clearly have some level of overlap. In any given area,
the infection may be most prevalent among users of intravenous drugs
who share needles. In another, the main risk group may be men who have
sex with other men. And in yet another, the main risk group may be
female sex workers. These sub-epidemics each follow their own
pattern, although there is some degree of interdependence. Nearly all
early cases of HIV infection detected in the Western Hemisphere were in
homosexual men, but female partners of bisexual men with HIV infection
gave rise to an increased spread among heterosexual persons.
Contributing to the increased cross-prevalence were persons with
hemophilia who had been infected with HIV from contaminated factor VIII
and persons who used intravenous drugs, an activity that transcends all
sexual preferences. Currently, less than half of new HIV infections are
reported in homosexual men, and infected heterosexual women outnumber
infected heterosexual men nearly two to one.[52] See the image below.

- Incidence
of HIV infection by risk group. From the CDC Web site (copyright free)
derived from the revised 2006 estimated figures. One
community-based study targeting areas where men who have sex with men
(MSM) meet demonstrated that an average of 44% of study participants
appeared unaware of their HIV-positive status. High rates of positivity
and unawareness of positive status were associated with younger
participants, men of Black non-Hispanic race, and lower education
levels. Healthcare visits in the last year were associated with a lower
rate of unawareness (37% vs 81%) but a higher rate of HIV-positivity
(21% vs 12%). Because this study targeted a high-risk group and may
involve participation bias, the overall rate of HIV infection (19%) can
not be easily extrapolated to the overall population.[53]
- International
The latest figures from the Joint United Nations Programme on HIV/AIDS are from 2008 (published in 2009).Worldwide,
approximately 33.4 million people (1% of the global adult population
aged 15-49 y) are infected with HIV, a decline from 2006 (39.5 million
reported at that time). UNAIDS estimates that 2.7 million people were
newly infected with HIV and that 2 million people died from AIDS in
2008, both statistics showing a slight decline over time. The vast
majority of infections remain in sub-Saharan Africa, where 5.2% of the
population is thought to be infected. Between 2004 and 2006, the
prevalence of HIV infection in central and eastern Asia and Eastern
Europe increased by 21%. During this period, the number of new HIV
infections in persons aged 15 to 64 years rose by 70% in Eastern Europe
and central Asia. The infection rates in many developed countries
remain stable, and some developing countries have achieved significant
gains in controlling and even reversing the effects of the HIV epidemic.
However, this is partially due to deaths in HIV-infected people,
together with simultaneous prevention of new infections. These figures
together show that global HIV infection is in a state of flux. The
mortality rate in some countries has greatly increased. In South Africa
(a country that, despite having a relatively late-onset HIV epidemic,
has developed one of the highest prevalence rates), the all-cause
HIV-associated mortality rate increased by 79% between 1997 and 2004. In
women aged 25-34 years, mortality rates increased by 500% during this
period. Swaziland has the highest overall prevalence of HIV infection (>26% of all adults based on 2007 figures).The
Ministry of Health in Zambia predicts that, without therapy and
assuming current levels of prevalence, young adults have a 50% lifetime
risk of dying from AIDS. In developing nations, co-infection with
HIV and tuberculosis is very common. The immunosuppressed state induced
by HIV infection contributes not only to a higher rate of tuberculosis
reactivation but also to an increased disease severity, as with many
other opportunistic infections. Further details of the global epidemic can be found in the Joint United Nations Programme on HIV/AIDS 2009 Epidemic Update.
- Mortality/Morbidity
Untreated
HIV infection carries an overall mortality rate of more than 90%. The
CD4 T-cell counts remain stable in a small percentage of people with HIV
infection. This is usually associated with strong anti-HIV CD8 T-cell
responses, a low viral load, and low proviral reservoir. The average
interval between initial HIV infection and progression to AIDS is 8-10
years. Once infection has progressed to AIDS, the survival period
is usually less than 2 years in untreated patients. Persons in whom the
infection does not progress long-term may not develop AIDS for 15 years
or longer, although many still exhibit laboratory evidence of CD4
T-cell decline or dysfunction.[54, 55, 56, 57] The
appropriate use of combination antiretroviral therapies and prophylaxis
for opportunistic infections dramatically improves survival and greatly
decreases the risk of secondary opportunistic infections.[58, 59, 60] The
risk of AIDS-associated lymphoma is not altered by antiviral therapy
and, as such, has grown in prevalence among overall AIDS-defining
conditions. Sackoff et al found that, since 1999, the HIV-related
mortality rate in New York City has decreased by approximately 50 deaths
per 10,000 people with AIDS per year. The rate of non–HIV-related
deaths has also seen a more modest but consistent decline, with about
7.5 fewer deaths per 10,000 people with AIDS per year.[59] Importantly,
many researchers have consistently shown that the primary risk factor
for infection affects mortality. For example, the mortality rate among
intravenous drug users tends to be higher, whether related to HIV
disease or non-HIV disease. Overall, with the increasing use of
antiretroviral therapy and the introduction of better antiviral
regimens, survival with HIV infection has increased over time, although
it is not yet equivalent to that in uninfected individuals. See the
image below.

- Changes
in survival of people infected with HIV. As therapies have become more
aggressive, they have been more effective, although survival with HIV
infection is not yet equivalent to that in uninfected people. Modified
from an original published by Lohse et al (2007), "Survival of persons
with and without HIV infection in Denmark, 1995-2005." In
addition to the concern for new opportunistic infections, pre-existing
infections can reactivate and cause significant disease in people with
AIDS. The most important example on a global scale is that of
tuberculosis, as reactivated tuberculosis can cause symptomatic disease
with lower levels of reactivation. Other important pathogens include
cytomegalovirus, (which causes retinitis, pneumonitis, and colitis) and Pneumocystis jiroveci (formerly known as Pneumocystis carinii; the causative organism in Pneumocystis
pneumonia). In immunocompetent hosts, these organisms are generally
nonpathogenic, and asymptomatic infection is common (and in the case of
cytomegalovirus infection, life-long). Antiviral medications are
associated with adverse effects and thus contribute to patient morbidity
and mortality rates, especially because of the growing population of
long-term survivors who are receiving combination antiviral therapy. In
particular, protease inhibitors may cause lipid-profile abnormalities.
- Race
In
the United States, the prevalence of HIV infection is highest in blacks
(71.3 cases per 100,000 population). The prevalence is also high among
Hispanic persons (27.8 per 100,000 population). These increased rates
are due to socioeconomic factors rather than genetic predisposition.
- Sex
In
the developed world, HIV infection is much more common in males. Among
heterosexuals, females are more likely to acquire HIV infection from an
infected male than a male is from an infected female, but a large
proportion of infections in males are due to homosexual contact, with or
without injection drug use. Males are also more likely to acquire HIV
infection from injection drug use alone. Males were also more
likely to acquire HIV infection through contaminated blood products
during treatment of hemophilia before universal testing of the blood
supply was instituted. (The procedures used in purifying factor VIII and
producing cryoprecipitate are effective in preserving biologic activity
of HIV. To negate this, heat treatment was added to the purification of
factor VIII to inactivate HIV and other viruses). This is a small
contribution to the predominance of HIV infection in males. In
the developing world, HIV infection is equally common in males and
females. The primary route of HIV transmission in the developing world
is heterosexual contact.
- Age
Young adults tend to be at
higher risk of acquiring HIV, typically through high-risk activities
such as unprotected sexual intercourse or intravenous drug use. A
study of neonatal immune responses from a cohort of HIV exposed infants
in South Africa demonstrated significantly lower antibody titers to
tetanus, Haemophilus influenza type B, pneumococcus, and pertussis compared to nonexposed infants.[61] This
suggests an effect of HIV on maternal antibody production and secondary
effects on transplacentally acquired IgG in HIV-exposed neonates. Vaccine
responses in these infants were robust; however, the implication is a
potentially higher risk of infectious disease in the newborn period,
even in the absence of vertically acquired HIV infection.



