
Medical Book
Buy Textbooks | Autoclaves | stethoscopes | Buy Books Online | Buy Medical Textbooks | Textbooks | Equipment | Nutrition | USMLE | MRCP | MRCS | Dental | Sport Medicine | Cardiology | Medical Textbook | Surgery | Pregnancy | Anatomy | Radiation | Pedia |
|
|
| | | Author | Message |
|---|
john

Membership NO : 1
 Posts : 1672 Posts : 1672
Join date : 2011-03-27
 |  Subject: Atrial Flutter Subject: Atrial Flutter  Wed Jun 08, 2011 1:21 pm Wed Jun 08, 2011 1:21 pm | |
| Atrial Flutter Atrial
Fibrillation,fibrillation,AF,Heart,ECG,Atrial
Fibrillation,cardiac,heart,Atrial Fibrillation,ECG,AF,ECG,Cardiac
muscle,AF,ECG,Atrial Fibrillation,AF,ECG,AF,ECG,Cardiac
muscle,AF,ECG,Atrial Fibrillation,AFBackgroundAtrial flutter has many clinical aspects that are similar to atrial fibrillation (ie, underlying disease, predisposing factors, complications, medical management). However, the underlying mechanism of atrial flutter makes it amenable to cure this arrhythmia with percutaneous catheter-based techniques. Some patients have both atrial flutter and atrial fibrillation. The elimination of atrial flutter has been noted to reduce or eliminate episodes of atrial fibrillation. Left untreated, persistent atrial flutter can degenerate into chronic atrial fibrillation. Uncommon forms of atrial flutter have been noted during long-term follow-up in as many as 26% of patients with surgical correction of congenital cardiac anomalies. Next Section: Pathophysiology PathophysiologyIn
most studies, approximately 30% of patients have no underlying cardiac
disease, 30% have coronary artery heart disease, and 30% have
hypertensive heart disease. Other conditions are also associated with
atrial flutter, including cardiomyopathy, hypoxia, chronic obstructive
pulmonary disease, thyrotoxicosis, pheochromocytoma, electrolyte
imbalance, and alcohol consumption. Animal models have been used to demonstrate that an anatomical block (surgically created) or a functional block of conduction between the superior vena cava and inferior vena cava, similar to the crista terminalis in the human right atrium, is key to initiating and maintaining the arrhythmia. In humans, the most common form of atrial flutter (type I [classic]) involves a single reentrant circuit with circus activation in the right atrium around the tricuspid valve annulus (most often in a counterclockwise direction), with an area of slow conduction located between the tricuspid valve annulus and the coronary sinus ostium (subeustachian isthmus).  Twelve-lead ECG of type I atrial flutter. Note negative sawtooth pattern of flutter waves in leads II, III, and aVF. A 3D electroanatomic map of type I atrial flutter:The 3-dimensional electroanatomic map of type I atrial flutter. The colors progress from blue to red to white and represent relative conduction time in the right atrium (early to late). An ablation line (red dots) has been created on the tricuspid ridge extending to the inferior vena cava. This interrupts the flutter circuit.RAA: right atrial appendage; CSO: coronary sinus os; IVC: inferior vena cava; TV: tricuspid valve annulus. The crista terminalis acts as another anatomic conduction barrier, similar to the line of conduction block between the 2 venae cavae required in the animal model. The orifices of both venae cavae, the eustachian ridge, the coronary sinus orifice, and the tricuspid annulus complete the barrier for the reentry circuit. Atrial flutter is often referred to as isthmus-dependent flutter. Usually the rhythm is due to reentry, there is an excitable gap, and the rhythm can be entrained. 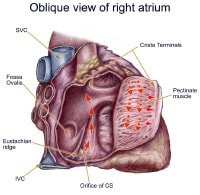 The anatomy of classic counterclockwise atrial flutter. This demonstrates an oblique view of the right atrium and shows some of the crucial structures. The isthmus of tissue responsible for atrial flutter is seen anterior to the orifice of the coronary sinus. The Eustachian ridge is part of the crista terminalis that separates the roughened part of the right atrium from the smooth septal part of the right atrium. Classic counterclockwise atrial flutter has caudocranial activation (ie, counterclockwise around the tricuspid valve annulus when viewed in the left antero-oblique fluoroscopic view) of the atrial septum. 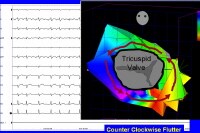 Classic counterclockwise atrial flutter. This 3-dimensional electroanatomic map of the tricuspid value and right atrial show the activation pattern displayed in color format. Red is early and blue is late relative to a fixed point in time. Activation travels in a counterclockwise direction. Classic atrial flutter can also have the opposite activation sequence (ie, clockwise activation around the tricuspid valve annulus). Clockwise atrial flutter is much less common. This arrhythmia is still considered type I, isthmus-dependent, clockwise flutter.Type II (atypical) atrial flutters are less extensively studied and electroanatomically characterized. Atypical atrial flutters may originate from the right atrium (surgical scars [ie, incisional reentry]) or from the left atrium (pulmonary veins [ie, focal reentry] or mitral annulus).  Atypical LA flutter. Left atrial flutter is common after incomplete left atrial ablation procedures and may result in faster ventricular rates than seen during atrial fibrillation. Thus, tricuspid isthmus dependency is not a prerequisite for atrial flutter. Often, the atrial rate is faster (340-350 bpm) in atypical flutter and the arrhythmia can not be entrained. Previous Next Section: Pathophysiology Epidemiology
FrequencyUnited StatesAtrial flutter is much less common than atrial fibrillation. From 1985-1990, of patients admitted to US hospitals with a diagnosis of supraventricular tachycardia, 77% had atrial fibrillation and 10% had atrial flutter. Based on a study of patients referred for tertiary care centers, the incidence of atrial flutter in the United States is estimated at 200,000 new cases per year. [1] Mortality/MorbidityPrognosis depends on the patient's underlying medical condition. Any atrial arrhythmia can cause a tachycardia-induced cardiomyopathy. Intervening to control the ventricular response rate or to return the patient to sinus rhythm is important. Thrombus in the left atrium has been described in patients with atrial flutter (0-21%). Thromboembolic complications have also been described. Due to conduction properties of the atrioventricular node, many people with atrial flutter will have a faster ventricular response (than those with atrial fibrillation). Heart rate is often more difficult to control with atrial flutter than with atrial fibrillation. SexAtrial flutter is associated with a male predominance. In a study of 100 patients with atrial flutter, 75% were men. In another study performed at a tertiary care study, atrial flutter was 2.5 times more common in men. AgePatients with atrial flutter, as with atrial fibrillation, tend to be older adults. In one study, the average age was 64 years (range 27-86 y).  |
|   | | john

Membership NO : 1
 Posts : 1672 Posts : 1672
Join date : 2011-03-27
 |  Subject: Re: Atrial Flutter Subject: Re: Atrial Flutter  Wed Jun 08, 2011 1:27 pm Wed Jun 08, 2011 1:27 pm | |
| Atrial Flutter Clinical PresentationHistoryThe severity of symptoms and the patient's underlying cardiac condition dictate the initial management approach. The most common symptom is palpitations. Other symptoms include fatigue, dyspnea, and chest pain.
- Address
symptoms of other noncardiac conditions (eg, hyperthyroidism, pulmonary
disease) or cardiac conditions associated with atrial flutter that may
be reversible.
- The most common symptom is palpitations. Other symptoms include fatigue, dyspnea, and chest pain.
- Assessing
the onset of symptoms/palpitations is critical. Atrial flutter (of a
duration >48 h) requires anticoagulation with warfarin or
transesophageal echo to rule out thrombus in the left atrium prior to
cardioversion to sinus rhythm. Thus, the duration of the episode and the
onset of atrial fibrillation or flutter may affect the timing of
cardioversion and the need to address anticoagulation.
- Precipitating causes and modes of termination of the arrhythmia.
- Previous response to pharmacologic therapy.
- Often,
atrial flutter is not as well tolerated as atrial fibrillation. This
may be due to the rapid and difficult-to-control ventricular response,
especially with minimal exertion.
- Atrial
flutter can cause hypotension, angina, congestive heart failure, and
rarely syncope due to rapid ventricular response in the setting of
compromised left ventricular function.
Physical
- The
general appearance and vital signs of the patient are important when
determining the urgency with which to restore sinus rhythm. Thus, the
initial cardiopulmonary evaluation and monitoring for signs of cardiac
or pulmonary failure help guide initial management.
- Evaluate the vitals with a close eye on heart rate, blood pressure, and oxygen saturation.
- Palpate the neck/thyroid gland for goiter.
- Evaluate the neck for jugular venous distention.
- Auscultate the lungs for rales/crackles.
- Auscultate/palpate the heart for extra heart sounds and murmurs, and palpate the point of maximum impulse.
- Examine the extremities to access for lower extremity edema/perfusion.
Causes
- Atrial
flutter is most often associated with left ventricular dysfunction,
rheumatic heart disease, congenital heart disease, and postcardiac
surgery.
- Thyroid disease, obesity, pericarditis,
pulmonary disease, and pulmonary embolism have been associated with
atrial fibrillation and atrial flutter. Rarely, mitral valve prolapse
has been associated with atrial flutter.
- Rarely, atrial flutter can be associated with an acute myocardial infarction.
- Postcardiac surgery, atrial flutter may be reentrant as a result of natural barriers, atrial incisions, and scar.
- Some patients develop atypical left atrial flutter after pulmonary vein isolation for atrial fibrillation.
- Atrial Flutter Differential Diagnoses
- Differentials
- Atrial Fibrillation
- Atrial Tachycardia
- Atrial Flutter Workup
- Laboratory Studies
- The
history and physical examination findings guide laboratory studies.
Asymptomatic hyperthyroidism, especially in elderly patients, can
manifest with atrial fibrillation or flutter; therefore, obtain thyroid
function tests. Hyperthyroidism is a rare cause of atrial flutter and
should be excluded with blood testing.
- Serum electrolytes and pulmonary function tests may be indicated based on the history.
- Electrocardiography is essential in making the diagnosis.
- The
common form of type I atrial flutter has sawtooth flutter (F) waves,
best seen in leads II, III, and aVF, with atrial rates of 240-340 bpm
and without an isoelectric interval between these F waves.
- The ventricular response may be regular or irregular.
- The ventricular rate is a fixed mathematical relationship of flutter waves and the resulting QRS complexes.
- Variable
AV conduction can also be seen (commonly present with 2:1 or 3:1 AV
conduction). With 1:1 AV conduction, hemodynamic collapse may occur.
- Morphology
of the flutter wave can predict findings in the electrophysiology
laboratory. A negative flutter wave in the inferior limb leads and a
positive flutter wave in V1 are highly predictive of a
counterclockwise circuit; however, with positive flutter waves in the
inferior limb leads and negative flutter waves in V1,
differentiating between clockwise type I atrial flutter and atypical
forms of non–isthmus-dependent intra-atrial
- reentry is difficult.
-

- Twelve-lead ECG of type I atrial flutter. Note negative sawtooth pattern of flutter waves in leads II, III, and aVF.
- Imaging Studies
- Transthoracic
echocardiography should be performed to evaluate for structural
abnormalities and left ventricular systolic function. It also can detect
valvular abnormalities, left ventricular hypertrophy, and pericardial
disease.
- Transesophageal echocardiography is the preferred technique to detect thrombus in the left atrium.
- Chest radiograph may be useful in evaluation of lung disease and the pulmonary vasculature.
- Procedures
See Medical Care for information regarding radiofrequency ablation and electrical cardioversion.
- Atrial Flutter Treatment & Management
- Medical Care
General
goals for the treatment of symptomatic atrial flutter are similar to
those for atrial fibrillation and include (1) control of the ventricular
rate, (2) restoration of sinus rhythm, (3) prevention and decreased
frequency or duration of recurrent episodes, (4) prevention of
thromboembolic complications, and (5) minimization of adverse effects
from therapy. However, these goals can be modified for each patient. In
an acute setting with pending hemodynamic collapse, follow the adult
advanced cardiac life support algorithms for managing atrial
fibrillation and flutter. Consider immediate electrical cardioversion
for patients who are hemodynamically unstable.The main difference
between atrial fibrillation and atrial flutter is that most cases of
atrial flutter can be cured with radiofrequency ablation. In all
available studies, catheter ablation is superior to rate control and
rhythm control strategies with antiarrhythmic drugs.
- Ventricular rate control
Ventricular
rate control is a priority because it may alleviate symptoms. Rate
control is typically more difficult for atrial flutter than for atrial
fibrillation.
- Calcium channel blockers and beta-blockers
- Ventricular
rate control can be achieved with drugs that block the AV node.
Intravenous calcium channel blockers (eg, verapamil, diltiazem) or
beta-blockers can be used, followed by initiation of oral agents.
- Hypotension and negative inotropic effects are concerns with the use of these medications.
- A
history of Wolff-Parkinson-White syndrome or evidence of ventricular
preexcitation should be determined because agents that act exclusively
at the level of the AV node may enhance accessory pathway conduction.
</li> Vagal maneuvers: These can be helpful in determining the underlying atrial rhythm if flutter waves are not seen well.
Intravenous
adenosine: This drug, administered as an intravenous push followed with
an intravenous bolus with flush, can also be helpful in making the
diagnosis of atrial flutter by transiently blocking the AV node.
Restoration of sinus rhythmAfter determining the patient's needs for anticoagulation and ventricular rate control, the issue of restoration of the sinus rhythm can be safely addressed.
- Radiofrequency ablation
- Radiofrequency
ablation is often used as first-line therapy to permanently restore
sinus rhythm. This procedure is often performed electively, rather than
in the acute setting, to restore sinus rhythm.
- For patients
with recurrent symptomatic atrial flutter that is proven to be
isthmus-dependent in the electrophysiologic laboratory, expect a success
rate of higher than 95% with current technology.
- Catheter ablation has been shown to significantly improve the quality of life in patients with atrial flutter.
- The
frequency of hospital admissions and emergency department visits and
the number of antiarrhythmic drugs administered are decreased
significantly after ablation.
- Activity capacity significantly improves in patients with preexisting LV dysfunction.
- Type
I atrial flutters (tricuspid valve isthmus dependent): Catheter
ablation is typically an outpatient procedure. The procedure involves
moderate sedation and accessing the femoral veins for catheter
insertion. The diagnosis of atrial flutter is confirmed using pacing
maneuvers and ablation is performed typically at 6:00 on the tricuspid
valve isthmus. A line of block is required to interrupt the circuit.
(see image below). Postablation pacing maneuvers can confirm that the
substrate required for the circuit has been modified. Recurrence is less
than 5%. Postprocedure anticoagulation with warfarin is usually
continued for 4-6 weeks.
- Classic
counterclockwise atrial flutter. This 3-dimensional electroanatomic map
of the tricuspid value and right atrial show the activation pattern
displayed in color format. Red is early and blue is late relative to a
fixed point in time. Activation travels in a counterclockwise direction.
- Type II atrial flutters
(non—isthmus dependent): These circuits are amenable to catheter
ablation, especially in centers with advanced mapping systems. The
ablation procedure is similar but may involve additional mapping of the
left atrium (via a trans-septal puncture). Success depends on localizing
the circuit and creating a line of block that includes an electrically
inert anatomic structure (ie, the mitral valve annulus). While success
should approach 95%, recurrence is more common and may also require the
use of antiarrhythmic agents for suppression.
</li> Electrical cardioversion
The success rate of electrical cardioversion is higher than 95%.
Factors
to consider include synchronization of shocks to R waves, adequate
sedation, and electrode position (apex anterior, apex posterior,
anteroposterior).
Atrial flutter generally requires less energy for conversion than atrial fibrillation, and as few as 50 joules may be necessary.
If
cardioversion is not successful with one electrode configuration,
switching may improve success. A second set of electrodes can be used
with tandem or simultaneous shocks.
Biphasic external waveform may be more effective in restoring sinus rhythm.
A
few points to remember about the cardioversion technique include a wide
electrode separation in the right anterior and left posterior position
(sandwiching the atria) (the more traditional location of pad location
[anterior and apical] will also work), the application of pressure on
paddles or electrodes to reduce thoracic impedance, and the placement of
electrode patches under or lateral to the breasts in women.
Risius
et al found that in external electrical cardioversion of atrial
flutter, anterior-lateral electrode positioning yields results superior
to those achieved with anterior-posterior positioning. In a randomized
trial, 96 patients (72 of them men), received sequential biphasic
waveform shocks using a step-up protocol consisting of 50, 75, 100, 150,
or 200 J. Compared with anterior-posterior positioning,
anterior-lateral positioning resulted in successful cardioversion with
less mean energy (65 +/- 13 vs 77 +/- 13 J, P = 0.001) and fewer mean shocks (1.48 +/- 1.01 vs 1.96 +/- 1.00, P
= 0.001). In addition, cardioversion occurred with the first 50 J shock
in 73% of patients when anterior-lateral positioning was used, versus
36% with the anterior-posterior electrode position (P = 0.001).[2]
</li> Pharmacological cardioversion
Flecainide[3] is only effective in approximately 10% of patients.
Dofetilide[4] is effective in 70-80% of patients. This drug should be initiated in an inpatient setting.
Ibutilide[5, 6, 7, 8] is
effective, converting recent-onset atrial flutter to sinus rhythm in
63% of patients with a single infusion. This is the only agent available
intravenously in the United States that can be used for cardioversion.
This drug must be given in a monitored setting due to risk of QT
prolongation and torsade de pointes. The patient should be monitored
with continuous ECG monitoring for at least 4 hours after the infusion.
Large
single oral doses of type IC antiarrhythmic agents, such as propafenone
(450-600 mg) or flecainide (200-300 mg), have also been shown to be
effective in converting recent-onset atrial fibrillation to sinus
rhythm. Their use in atrial flutter can be assumed to have at least
equal success.
Combination of the above treatments:
Antiarrhythmic medication prior to electrical cardioversion has been
shown to improve the rate of conversion to sinus rhythm.
</li> Prevention (decrease frequency or duration of recurrence episodes)After the initial episode is terminated and the underlying disease is treated, the patient may not need any further intervention except avoidance of the precipitating factor (eg, alcohol, caffeine). For atrial fibrillation, approximately 30% of patients remain in sinus rhythm at 1 year without antiarrhythmic therapy.
- Antiarrhythmic agents
- For more information on the use of antiarrhythmic agents, see Atrial Fibrillation.
Data on the use of antiarrhythmic agents specifically for atrial
flutter are limited. Most studies of antiarrhythmics agents and atrial
fibrillation include some patients with atrial flutter (10-20%).
- In
general, the use of antiarrhythmic drugs in atrial flutter is similar
to that of atrial fibrillation; however, with a high success rate and
low complication rate, the use of radiofrequency ablation in atrial
flutter makes this procedure a favorable option compared with lifelong
antiarrhythmic drug therapy because fatal proarrhythmic events (even in
healthy hearts) and organ toxicity may occur.
- In general,
antiarrhythmics used to treat atrial fibrillation have been shown
effective in fibrillation or flutter during a 6 to 12 month follow-up.
Considering the characteristic adverse effects of each antiarrhythmic
agent, some guidelines are available regarding the choice of medication
when taking into account the underlying cardiac pathology.
- For patients without structural heart disease, class IC agents can be used safely.
- For patients with LV hypertrophy without ischemia or conduction delay, class III agents, specifically amiodarone, can be used.
- For patients with ischemic heart disease, sotalol or amiodarone can be used. Avoid class IC agents.
- For
patients with significant systolic dysfunction, amiodarone can be used,
dofetilide may be used, and class IC agents should be avoided.
</li> Surgery:
In patients who have atrial flutter and need cardiac surgery,
modification of the atrial incision and creation of a cryothermal
lesion, similar to the lesion created during radiofrequency catheter
ablation, can be curative for atrial flutter and may prevent an
incisional reentrant arrhythmia.
Prevention of complications
- Thromboembolic
- Patients
with atrial flutter are at increased risk of thromboembolic
complications compared with the general population. The anticoagulation
strategy used for atrial fibrillation is also recommended for atrial
flutter.
- In general, when atrial flutter persists for more than
48 hours, 4 weeks of adequate anticoagulation or TEE is needed before
attempting cardioversion to sinus rhythm.
- Thromboembolic
complications occur spontaneously after cardioversion or ablation, and
postconversion anticoagulation is recommended for a minimum of 4 weeks.
- Use
long-term anticoagulation for patients with persistent or paroxysmal
atrial flutter. As with atrial fibrillation, keep the international
normalized ratio (INR) at 2-3 to optimize the therapeutic effect and
minimize the risk of bleeding.
- Unlike atrial fibrillation,
atrial flutter has a regular pattern of atrial contraction. TEE data
have demonstrated an organized sawtooth pattern of the left atrial
appendage flow with alternating filling and emptying wavelets. No
difference in the left atrial appendage function is observed compared
with patients in sinus rhythm. Patients with both atrial flutter and
atrial fibrillation have significantly decreased left atrial appendage
function, more spontaneous echo contrast, and larger left atria and
accompanying appendages.
- Patients with atrial flutter and
episodes of atrial fibrillation are at higher risk of thromboembolic
events; however, determining whether episodes of atrial fibrillation are
associated with episodes of atrial flutter is difficult.
- A
large retrospective review of patients in chronic atrial flutter
revealed a 14% occurrence rate of thromboembolic events over 4.5 years,
with half of these events being ischemic stroke. In another large cohort
of patients with atrial flutter, the occurrence rate of embolic
complications in patients with chronic or recurrent atrial flutter was
12%. For stroke, this risk is estimated at approximately one third of
patients with nonrheumatic atrial fibrillation. Males with hypertension,
structural heart disease, LV dysfunction, and diabetes may be at higher
risk of thromboembolic complications. Interestingly, associated atrial
fibrillation did not significantly increase the risk of the embolic
complications.
- Other reports have demonstrated thrombus in the
left atrium appendage of patients with atrial flutter (as many as 43%).
Most studies of non–anticoagulated patients with atrial flutter report a
rate of 10-15% for patients with thrombus in the left atrium or left
atrial appendage. Spontaneous echo contrast associated with increased
risk of thromboembolism was found in 6-43% of patients with atrial
flutter.
- The CHA2DS2-VASc score includes congestive heart
failure, hypertension, age 65-74 years, diabetes, previous stroke,
vascular disease, and sex category. This score has been shown to perform
well at predicting patients at high-risk and patients categorized at
low risk for thromboembolism.[9]
- Postcardioversion
thromboembolic events can complicate as many as 7.3% of procedures in
patients who are not anticoagulated. These events occur within 3 days
after the cardioversion; almost all occur within 10 days after the
cardioversion[10] .
- In atrial fibrillation, postcardioversion stunning of the left atrial appendage is thought to contribute to thrombogenicity.[11] This
phenomenon may last as long as 4 weeks in patients with atrial
fibrillation and may be related to how long patients have been in
arrhythmia.
- Stunning of the left atrial appendage also occurs
following conversion from atrial flutter to sinus rhythm (electrical or
spontaneous), although to a lesser degree. Left atrial and left atrial
appendage function decrease immediately after conversion, and, in one
study, spontaneous echo contrast was noted to develop within 5 minutes
after conversion in 43% of patients. This is thought to be the source of
emboli in patients whose TEE findings revealed no evidence of thrombus
but who had a thromboembolic event after cardioversion.
- In a
study comparing left atrial appendage function before and after catheter
ablation (immediate, 1 d, 1 wk, and 6 wk) of persistent atrial flutter,
a significant increase in atrial standstill, decrease in left atrial
appendage function, and new spontaneous echo contrast occurred after
ablation. One patient formed a new left atrial appendage thrombus after
ablation. Evidence of atrial stunning significantly improved after 1
week. Anticoagulation for at least 1 week is advocated after ablation of
an atrial flutter that persists for more than 2 days.
- Adequate anticoagulation, as recommend by the American College of Chest Physicians,
has been shown to decrease thromboembolic complications in patients
with chronic atrial flutter and in patients undergoing cardioversion.
</li> Cardiomyopathy:
Termination of long-standing atrial flutter with a rapid ventricular
response has been reported to improve LV systolic function in patients
without other known causes of dilated cardiomyopathy.
Minimizing adverse effects of antiarrhythmic therapyBecause atrial flutter is a nonfatal arrhythmia, carefully assess the risks and benefits of drug therapy, especially with antiarrhythmic agents. Always consider catheter-based ablation as first-line therapy prior to starting an antiarrhythmic agent. A few points to remember that will help minimize the adverse effects include the following:
- Avoidance
of precipitating factor(s) or therapy of the underlying problem may be
all that is needed to prevent recurrent episodes.
- Of
antiarrhythmic agents, amiodarone is effective and is associated with a
low proarrhythmic risk but may adversely affect multiple organs,
including the skin, liver, lungs, and thyroid. Thus, sotalol would seem a
reasonable choice of antiarrhythmic drug therapy for atrial flutter.
Per guidelines, sotalol should be initiated in the inpatient setting.
- Radiofrequency
ablation is currently the preferred therapeutic choice. Although many
patients who were treated with radiofrequency ablation subsequently
developed atrial fibrillation after long-term follow-up (56% in one
study), this procedure still represents a safe alternative to
antiarrhythmic agents.
Next Section: Consultations Consultations
In general, consult a cardiologist and/or electrophysiologist because the use of antiarrhythmic drugs may be harmful and radiofrequency ablation may eliminate atrial flutter. Previous Next Section: Consultations DietAny dietary recommendations should be appropriate for the underlying heart disease and other comorbidities (eg, diabetes). </li>
Atrial Flutter Medication
Medication Summary
Medications
are usually used in the acute setting or in people who are not
candidates for radiofrequency ablation. Agents can be used to control
the ventricular rate, terminate acute episodes, prevent or decrease the
frequency or duration of recurrent episodes, and prevent complications.
Various categories of drugs are used to treat atrial flutter. Drug
initiation in an outpatient setting is generally accepted in patients
without underlying structural heart disease who are in sinus rhythm. In
addition, many specialists initiate outpatient drug therapy in patients
with therapeutically anticoagulated atrial flutter who are awaiting
outpatient electrical cardioversion in the near future. Certain
medications, such as initiation of sotalol and dofetilide, by guidelines
should be administered in an inpatient setting as they can prolong the
QT interval and be proarrhythmic. Regardless, close patient follow-up is
mandated, with frequent ECG monitoring or transtelephonic monitoring
for potential signs of proarrhythmia.
Next Section: Atrioventricular nodal conduction blockers
Atrioventricular nodal conduction blockers
Class Summary
Used
to slow ventricular response by slowing AV nodal conduction during
atrial fibrillation or flutter. Also indicated for use in conjunction
with class IA and IC antiarrhythmics, which slow atrial
fibrillation/flutter rate and may cause more rapid ventricular response.
View full drug information
Metoprolol (Lopressor)
Selective
beta1-adrenergic receptor blocker that decreases automaticity of
contractions. During IV administration, carefully monitor BP, heart
rate, and ECG. View full drug information
Atenolol (Tenormin)
Selectively blocks beta-1 receptors with little or no effect on beta-2 receptors.View full drug information
Esmolol (Brevibloc)
Excellent
for use in patients at risk for experiencing complications from
beta-blockade, particularly those with reactive airway disease,
mild-to-moderate LV dysfunction, and/or peripheral vascular disease.
Short half-life of 8 min allows titration to desired effect and quick
discontinuation if needed.
Previous
Next Section: Atrioventricular nodal conduction blockers
Calcium channel blockers (nondihydropyridine)
Class Summary
Effective for rate control.View full drug information
Verapamil (Calan)
Calcium
channel blocker. Only nondihydropyridines are effective for rate
control. During depolarization, inhibits calcium ions from entering slow
channels and voltage-sensitive areas of vascular smooth muscle and
myocardium. View full drug information
Diltiazem (Cardizem)
Only
nondihydropyridines are effective for rate control. During
depolarization, inhibits calcium ions from entering slow channels and
voltage-sensitive areas of vascular smooth muscle and myocardium.
Previous
Next Section: Atrioventricular nodal conduction blockers
Cardiac glycosides
Class Summary
AV nodal blocking agents.View full drug information
Digoxin (Lanoxin)
Slows
sinus node and AV node via vagomimetic effect and not very effective if
sympathetic tone is increased. Generally not recommended unless
depressed LV function is present.
Previous
Next Section: Atrioventricular nodal conduction blockers
Antiarrhythmics, class IC
Class Summary
For
use in patients with atrial flutter and SVT without structural heart
disease. Use in conjunction with AV nodal blocking agents when
administered to patients in atrial flutter because conversion to atrial
flutter with 1:1 conduction (producing fast ventricular rates) is noted.
View full drug information
Propafenone (Rythmol)
Treats
life-threatening arrhythmias. Possibly works by reducing spontaneous
automaticity and prolonging refractory period. Indicated for patients
with AF and SVT without structural heart disease. Use in conjunction
with AV nodal blocking agents when administered to patients in AF
because conversion to AFL with 1:1 conduction (producing fast
ventricular rates) is noted. View full drug information
Flecainide (Tambocor)
Treats
life-threatening ventricular arrhythmias. Causes prolongation of
refractory periods and decreases action potential without affecting
duration. Blocks sodium channels, producing a dose-related decrease in
intracardiac conduction in all parts of heart, with greatest effect on
the His-Purkinje system (HV conduction). Effects on AV nodal conduction
time and intra-atrial conduction times, although present, are less
pronounced than on ventricular conduction velocity. Use in conjunction
with AV nodal blocking agents when administered to patients in AF
because conversion to AFL with 1:1 conduction (producing fast
ventricular rates) is noted.
Previous
Next Section: Atrioventricular nodal conduction blockers
Antiarrhythmics, class III
Class Summary
Class
III drugs widely used in maintenance of sinus rhythm in patients with
atrial flutter. Drugs may include amiodarone (Cordarone), sotalol
(Betapace), ibutilide (Corvert), and dofetilide (Tikosyn). View full drug information
Amiodarone (Cordarone)
May
inhibit AV conduction and sinus node function. Prolong action potential
and refractory period in myocardium and inhibit adrenergic stimulation.Prior to administration, control ventricular rate and CHF (if present) with digoxin or calcium channel blockers.View full drug information
Sotalol (Betapace)
Class
III antiarrhythmic agent, which blocks K+ channels, prolongs action
potential duration (APD), and lengthens QT interval. Noncardiac
selective beta-adrenergic blocker. Sotalol is shown to be effective in
the maintenance of sinus rhythm, even in patients with underlying
structural heart disease. View full drug information
Ibutilide (Corvert)
Newer
class III antiarrhythmic agent that may work by increasing action
potential duration and thereby changing atrial cycle length variability.
Mean time to conversion is 30 min. Two thirds of patients who converted
were in sinus rhythm at 24 h. Ventricular arrhythmias occurred in 9.6%
of patients and mostly were PVCs. The incidence of torsades de pointes
was < 2%. View full drug information
Dofetilide (Tikosyn)
Recently
approved by FDA for maintenance of sinus rhythm. Increases monophasic
action potential duration, primarily due to delayed repolarization.
Terminates induced re-entrant tachyarrhythmias (eg, atrial
fibrillation/flutter, ventricular tachycardia) and prevents their
reinduction.Has no effect on cardiac output, cardiac index,
stroke volume index, or systemic vascular resistance in patients with
ventricular tachycardia, mild to moderate CHF, angina, and either normal
or reduced LVEF. No evidence of negative inotropic effect.
Previous
Next Section: Atrioventricular nodal conduction blockers
Antiarrhythmic agent, miscellaneous
Class Summary
Dronedarone is an antiarrhythmic agent with properties belonging to all 4 Vaughn-Williams antiarrhythmic classes.View full drug information
Dronedarone (Multaq)
Blocks
sodium channels, blocks beta1-adrenergic site, and alters adenyl
cyclase generation (ie, negative inotropic effects); blocks potassium
channels (eg, hERG) and therefore prolongs cardiac repolarization.In
a multinational clinical trial (n >4600), dronedarone reduced
cardiovascular hospitalization or death from any cause by 24% compared
with placebo.Indicated to reduce risk for cardiovascular
hospitalization in patients with paroxysmal or persistent atrial
fibrillation (AF) or atrial flutter (AFL), with a recent episode of
AF/AFL and associated cardiovascular risk factors (ie, age >70 y,
hypertension, diabetes, history of CVA, LAD >50 mm or LVEF < 40%)
who are in sinus rhythm or who will be cardioverted.
Previous
Next Section: Atrioventricular nodal conduction blockers
Anticoagulants
Class Summary
Used to prevent thromboembolic complications.View full drug information
Heparin
Augments
activity of antithrombin III and prevents conversion of fibrinogen to
fibrin. Does not actively lyse but is able to inhibit further
thrombogenesis. Prevents reaccumulation of clot after spontaneous
fibrinolysis. Most data are related to use of unfractionated heparin.
Low–molecular-weight heparin probably as effective but awaits results
from clinical studies. View full drug information
Warfarin (Coumadin)
Interferes
with hepatic synthesis of vitamin K–dependent coagulation factors. Used
for prophylaxis and treatment of venous thrombosis, pulmonary embolism,
and thromboembolic disorders.Tailor dose to maintain INR of 2-3.
Atrial Flutter Follow-up
Further Inpatient Care
Consider
catheter-based ablation as first-line therapy in patients with type I
typical atrial flutter if they are reasonable candidates. Ablation is
usually done as an elective procedure; however, it can be done when the
patient is in atrial flutter as well.
Given the high success rate and low complication rate, radiofrequency ablation is superior to medical therapy.
For
atrial flutter of less than 48 hours in duration, attempt cardioversion
as soon as possible. Postconversion anticoagulation is usually
unnecessary, although data from TEE studies indicate that postconversion
anticoagulation a reasonable option.
For episodes
of atrial flutter of uncertain duration or greater than 48 hours, begin
anticoagulation therapy. If cardioversion is needed sooner,
anticoagulate patients with intravenous heparin and perform TEE as close
to the time of cardioversion as possible. Patients still require
anticoagulation for at least 4 weeks after cardioversion.
If
thrombus is observed or suspected based on TEE findings, delay
cardioversion. Rate control and therapeutic anticoagulation is required
for a minimum of 4 weeks.
In patients who are not
candidates for catheter-based ablation, rate and rhythm control
strategies should be considered. The risk of proarrhythmia is probably
greatest during the first 24-48 hours after the initiation of
antiarrhythmics and drugs such as ibutilide, sotalol, and dofetilide
should be initiated in an inpatient setting. Pause-dependent torsades de pointes can occur after conversion to sinus rhythm.
Further Outpatient CareClosely monitor the patient's anticoagulation therapy, with a target INR of 2-3. Take special care when additional medications (including antibiotics) are added because they may cause dramatic alterations in INR values. Previous Next Section: Further Outpatient Care Inpatient & Outpatient Medications
- Anticoagulant
therapy (ie, heparin and/or warfarin) is indicated, especially when the
onset of atrial flutter is of more than 48 hours' duration or is
uncertain.
- Patients need to maintain a therapeutic INR for 3
weeks prior to conversion and for at least 4 weeks after conversion to
sinus rhythm.
- Long-term anticoagulation is recommended for patients with chronic atrial flutter.
- Anticoagulants are used to decrease thromboembolic complications.
</li> Preferred
medications that slow AV node conduction include beta-blockers (eg,
atenolol, metoprolol, propranolol) and calcium channel blockers (eg,
verapamil, diltiazem).
These medications are used to control ventricular rates.
Also
use these medications in patients who are taking class IA or IC
antiarrhythmic drugs (to prevent rapid ventricular response, which can
occur when the atrial rate is slowed).
</li> Antiarrhythmic drugs are indicated for the termination of acute episodes or the prevention of recurrent episodes.
For atrial flutter, electrical cardioversion is effective and usually requires less energy than for atrial fibrillation.
Catheter ablation offers a potential cure and is safer long-term use of an antiarrhythmic agent.
</li> ComplicationsThe major potential complication with atrial flutter (or atrial fibrillation) is neurologic insult, either transient ischemic attack or stroke. This risk can be minimized with proper anticoagulation. Consider patients with common type I atrial flutter for catheter ablation to eliminate the need for long-term anticoagulation and antiarrhythmic medications. Previous Next Section: Further Outpatient Care Prognosis
- Atrial
flutter itself is not considered a life-threatening arrhythmia;
however, uncontrolled ventricular rates can lead to impaired ventricular
function. Additionally, patients with Wolff-Parkinson-White syndrome
can develop life-threatening ventricular responses. Consider these
patients for catheter ablation of their accessory bypass tract. Data
from the Framingham study suggest that patients with atrial fibrillation
do not live as long as patients without atrial fibrillation (ie,
controls). No data are available on atrial flutter.
- The
prognosis for patients with Type I atrial flutter who undergo catheter
ablation is excellent, with a very low recurrence rate. The picture is
not as clear for patients with both atrial flutter and atrial
fibrillation. Some reports have documented fewer episodes of atrial
fibrillation after successful flutter ablation, while others have not.
Atrial fibrillation is thought to possibly be more responsive to
antiarrhythmic agents after atrial flutter has been eliminated.
- Numerous
reports indicate that patients with atrial fibrillation who are given
class IC antiarrhythmic agents may convert to atrial flutter with faster
ventricular rates. Thus, patients receiving type IC agents (flecainide)
should also receive an AV nodal blocking drug such as a beta-blocker or
calcium channel blocker.
Previous Next Section: Further Outpatient Care Patient Education
- Patient education regarding medications and diet is important.
- Patients
taking warfarin should avoid major changes in their diet unless
consulting with their providers. Recall that warfarin inhibits vitamin K
synthesis and that sources of vitamin K are green leafy vegetables. A
sudden change to a diet high in vitamin K may increase the requirements
for warfarin.
</li> </li> </li>  |
|   | | |
| | Permissions in this forum: | You cannot reply to topics in this forum
| |
| |
|
|






