Current Management of Patients With Metastatic Brain Tumors
Introduction Metastatic brain tumors continue to be the
most common intracranial tumor in adults. The prognosis for patients
with brain metastasis remains poor. Median survival for an untreated
patient is only about 1 month
[1] and increases to 4 months with steroids and whole-brain radiation therapy (WBRT).
[2] The most common sources of brain metastases are listed in the Table.
[1] Table. Most Common Sources of Brain Metastases | Lung cancer | ~ 50% |
| Breast cancer | 15%-20% |
| Melanoma | 10%-15% |
| Tumors of unknown origin | 10%-15% |
| Colorectal cancer | 2%-12% |
| Kidney cancer | 1%-8% |
| Thyroid primaries | 1%-10% |
Brain metastases are heterogeneous in terms of biology, response to
treatment, and prognosis. The Radiation Therapy Oncology Group (RTOG)
analyzed 1200 patients from 3 consecutive RTOG trials and by using
recursive partitioning analysis (RPA), established 3 prognostic groups.
[3] Patients in RPA class 1 (< 65 years of age with Karnofsky
performance score [KPS] ≥ 70 and a controlled primary tumor with the
brain as the only site of metastasis) had a median survival of 7.1
months. The worst survival, a median of 2.3 months, was seen in patients
within RPA class 3 (KPS < 70). All other patients are in RPA class
2, with a median survival of 4.2 months. Recently, other prognostic
classifiers, such as the graded prognostic assessment (GPA) and
subsequently a disease-specific GPA have been developed, and current
work is focused on developing patient-specific nomograms to predict
survival.
[4] Initial Medical Management The initial medical management of a patient
presenting with brain metastases includes the use of corticosteroids,
commonly dexamethasone, to reduce peritumoral edema and intracranial
pressure. Results are typically seen in a matter of hours in most cases.
However, the sustainability of this effect is limited, and given the
potential for substantial toxicity with high-dose dexamethasone, the
drug should be restricted to patients who clinically require steroids.
The duration and dosage should be tailored for each patient, with rapid
tapering considered for almost all patients.
[5] Similarly, use of anticonvulsants should be judicious. Because of the
absence of clear data showing that these drugs prevent seizures in a
patient who has not had a seizure, such use is generally not
recommended.
[6] Radiation Therapy and Surgery
WBRT As first described by Chao and colleagues in 1954,
[7] WBRT produced symptomatic relief in 63% of patients. It is the primary
palliative therapy for patients with multiple brain metastases; its use
prolongs median survival from 1-2 months to 3-7 months.
[7] Several randomized trials of radiation
therapy schedules done by the RTOG have not demonstrated the superiority
of one specific schedule over another. In an effort to balance risk and
benefit, the most commonly used regimens are 30 Gy in 10 fractions or
37.5 Gy in 15 fractions.
Harwood and Simpson
[8] randomly assigned 108 patients to 10 Gy in a single fraction or 30 Gy
in 10 fractions and found no significant difference in median survival
or recurrence. RTOG 6901 randomly assigned 993 patients to 1 of 4
treatment regimens: 30 Gy in 2 weeks, 30 Gy in 3 weeks, 40 Gy in 3
weeks, or 40 Gy in 4 weeks. RTOG 7361 randomized 1001 patients to 20 Gy
in 1 week, 30 Gy in 2 weeks, and 40 Gy in 3 weeks.
[9] Response, duration of improvement, and time to progression were
comparable across all arms. Other randomized trials have examined
hypofractionated schedules, such as 20 Gy in 5 fractions compared with a
standard-dose fractionation schedule (30 Gy in 10 fractions) and also
observed no significant differences between groups.
WBRT Plus Surgery Recent advances in neurosurgical techniques have reduced mortality and morbidity rates and have resulted in superior outcomes.
[10] Indications for surgery include the need for establishing pathologic
confirmation or if urgent decompression is needed to prevent herniation
and eliminate disabling symptoms. In addition, some reports contend that
for so-called "radioresistant" melanoma and renal cell metastases, the
effect of WBRT alone is minimal, thereby making surgery a more
attractive choice for these patients.
[11] Three older randomized trials compared WBRT
alone with WBRT plus surgery in patients with single brain metastases.
Patchell and colleagues
[12] randomly
assigned 48 patients with a single brain metastasis and a KPS of 90 to
resection followed by WBRT vs biopsy and WBRT. Local control, overall
survival, and functional independence were significantly superior in the
surgery arm. Similarly, Vecht and colleagues
[13] randomly assigned 63 patients to WBRT alone vs WBRT plus surgery and
found that patients who had resection had superior survival and
functional independence. Finally, Mintz and colleagues
[14] randomly
assigned 84 patients with a median age of 59 years and KPS > 50 to
surgery plus WBRT vs WBRT alone. In contrast to the others, this study
did not show any benefit of adding surgery, as many of the patients died
early from systemic progression.
These trials suggest that outcomes, including
survival, improve with resection of single brain metastases in
well-selected patients. Data from retrospective studies suggest that
resection of multiple brain metastases could have a survival benefit,
[15] but this has not been confirmed in a prospective randomized trial.
WBRT and Stereotactic Radiosurgery Stereotactic radiosurgery (SRS) delivers
highly conformal radiation to a well-defined, discrete, relatively small
lesion in a single large fraction, with maximum sparing of surrounding
tissues. The primary advantages of SRS are the ability to treat small
lesions (< 3 cm) in eloquent areas where surgery is not optimal, to
treat residual disease after surgical resection, or to avoid surgery in
patients with comorbid conditions. Local control rates for SRS have
ranged from 65%-94%, with an associated 5%-10% risk for radionecrosis.
[16-19] RTOG 90-05
[20] established the maximum tolerated doses for SRS as 24, 18, and 15 Gy
for tumors ≤ 2 cm, 2.1-3 cm, and 3.1-4 cm, respectively. Randomized
trials have since shown the benefit of SRS in conjunction with WBRT. For
example, in RTOG 9508,
[21] 333 patients with 1-3 metastases were randomly assigned to WBRT alone
or WBRT followed by SRS. Patients undergoing SRS were more likely to
have stable or improved performance status at 6 months (43% vs 27%), and
survival was improved with SRS for patients with a single brain
metastasis (6.5 months vs 4.9 months). Post hoc analysis showed that in
patients with a favorable prognosis, defined as KPS ≥ 70, controlled
primary tumor, and age < 65 years, SRS also improved survival (11.6
months vs 9.6 months).
SRS and WBRT are widely used in patients with
multiple brain metastases; however, their precise value remains
controversial because randomized prospective trials have shown no
survival benefit. Retrospective trials have suggested that patients with
multiple metastases may benefit from SRS, especially in the context of
local control.
[22,23]
SRS vs Resection No completed trial has compared SRS with
resection, but several nonrandomized comparisons suggest equivalence and
at least 1 recent case-matched analysis contends that SRS is superior.
[24] Adjuvant WBRT Following SRS or Surgery< Adjuvant WBRT after surgery or SRS has level 1
evidence to support its role, and yet it remains controversial,
primarily due to neurotoxicity concerns. Although recurrence rates are
clearly lower following WBRT, an overall survival benefit has not been
categorically demonstrated.
[25-28] The role of WBRT following surgery or SRS in
patients with 1-3 metastases was most extensively evaluated in a trial
conducted by the European Organization for Research and Treatment of
Cancer (EORTC 22952-26001).
[25] In this trial, 359 patients with 1-3 brain metastases were randomly
assigned to WBRT or observation following either SRS (n = 199) or
surgery (n = 160). At 2 years, significantly fewer patients had
intracranial progression after WBRT, either at the original site or at
new locations (31% vs 54%), but there was no difference in overall
survival (median 10.7 months vs 10.9 months).
DeAngelis and colleagues
[29] retrospectively reviewed 47 patients with brain metastasis treated with
WBRT and found an 11% risk for dementia in long-term survivors (>12
months). Nearly all patients who developed dementia received nonstandard
WBRT schedules, and none of the 15 patients who received standard dose
schedules developed dementia. In contrast, a phase 3 trial by the
Japanese Radiotherapy Oncology Group evaluated patients treated with SRS
followed by immediate WBRT vs SRS alone and found no difference in
neurologic function; in fact, time to deterioration in Mini-Mental
Status Examination score was shorter in the SRS-alone group, implying
that the consequences of intracranial recurrence could be worse than
those seen with WBRT.
[27] More recently, a small, single-institution
trial found a greater likelihood of deterioration in the 4-month Hopkins
Verbal Learning Test-Revised (HVLT-R) in patients receiving WBRT with
SRS vs SRS alone.
[28] This finding is not completely surprising, as the 4-month mark is
believed to represent the peak time point for manifestation of post-WBRT
fatigue, oligodendroglial cell death, and loss of neurogenic
differentiation from the perihippocampal stem-cell compartment. However,
the 4-month mark is also the time point at which it is early to see the
benefits of WBRT in reducing intracranial relapses that lead to
neurocognitive decline
[30] -- making it, in effect, a "perfect storm" time point.
The question of balance of neurocognitive
function relative to WBRT plus SRS vs WBRT alone is currently being
addressed in the context of an intergroup randomized trial.
[31] Surgery Followed by SRS In an attempt to withhold WBRT, SRS has been
used after surgery, primarily to reduce local relapse rates, which can
run as high as 30% or more following resection. Although there are no
randomized data to validate a benefit for resection of brain metastasis
followed by a radiosurgery boost, several centers follow this approach.
Our own unpublished institutional data show a median survival of 53
months in 57 well-selected patients with single brain metastases treated
with SRS to the resection bed, with < 10% local recurrence in the
vicinity of the resection cavity and 37% recurrence in distant
intracranial sites. These results are encouraging but need to be
confirmed in a randomized trial.
Brachytherapy Brachytherapy involves implantation of
radioactive sources directly into an intracerebral mass or surgical
cavity, allowing the most conformal type of radiotherapy. This allows
for delivery of potentially higher radiation doses than with external
beam therapy, while still limiting the dose of radiation to the
surrounding brain.
Brachytherapy has been used in combination
with resection, as definitive therapy in selected patients with
unresectable metastases and after recurrence following previous WBRT or
surgery. Although brachytherapy has been largely replaced by SRS for
small lesions, there still may be a potential role for lesions that are
too large for SRS.
[32] In 1 study of 93 patients, most of whom had solitary metastases, 38
patients received brachytherapy in conjunction with external beam
radiotherapy, 34 received brachytherapy alone, and 21 received
brachytherapy after previous radiotherapy or surgery had failed.
[33] Median survival for the 3 groups was 17, 15, and 6 months, respectively.
A newer strategy for brachytherapy involves
the use of a photon radiosurgery system. A miniature x-ray generator
with an attached probe is placed stereotactically into the metastasis at
the time of craniotomy to deliver a single fraction of high-dose
radiation (6 to 15 Gy) over less than 1 hour.
[34] Another alternate form of brachytherapy uses an inflatable balloon catheter containing a liquid I-125 radioisotope (GliaSite
®; Hologic Inc., Bedford, MA) inserted at the time of resection of a metastatic lesion.
[35] Experience with both the photon radiosurgery system and GliaSite in this setting is limited.
Approaches for Reducing Cognitive Dysfunction Most patients undergoing WBRT already have
cognitive deficits prior to therapy; 1 phase 3 study reported baseline
cognitive impairment in 91% of patients.
[36] Therefore, posttreatment analysis without correcting for pretreatment
impairment often provides a highly misleading assessment. Modern trials
correct for these factors, and strategies are being actively pursued to
reduce cognitive dysfunction. With regard to pharmacologic intervention,
agents such as methylphenidate, memantine, and donepezil, have shown
initial promise.
[37-39] Modifications in WBRT to spare neurogenic stem-cell compartments are
also being studied. The perihippocampal neurogenic stem-cell compartment
is very sensitive to radiation, and avoidance of this zone with
conformal radiotherapy techniques is under active investigation.
[40,41] Note that patients receiving > 3 Gy per fraction may be at greater
risk for cognitive decline, so high doses should be avoided.
[29] Radiation Sensitizers
Motexafin Gadolinium Motexafin gadolinium (MGd) selectively
concentrates in tumors, where it depletes agents needed for repair of
cytotoxic damage, thereby enhancing radiation-induced apoptosis.
[42,43] In a phase 3 trial of 401 patients with brain metastases from various
primary tumors, the addition of MGd to radiotherapy significantly
lengthened the time to neurologic and/or cognitive decline in patients
with non-small cell lung cancer [(NSCLC); see Figure].
[36,43] 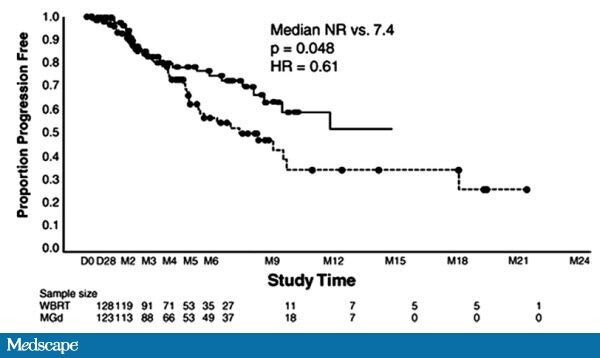 Figure.
Figure. Time to neurologic
progression by treatment arm. From Mehta MP, et al. Survival and
Neurologic Outcomes in a Randomized Trial of Motexafin Gadolinium and
Whole-Brain Radiation Therapy in Brain Metastases.
J Clin Oncol. 2003;21(13):2529-2536. Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved.
A confirmatory phase 3 trial randomized 554 patients to WBRT or WBRT
plus MGd and reported longer neurologic progression-free survival in
patients receiving prompt WBRT and MGd (15.4 months vs 10 months).
[44] In particular, patients who received WBRT within 3 weeks of diagnosis
of brain metastases had a significant improvement in time to neurologic
progression.
Efaproxiral Hypoxic tumor cells are more resistant to
radiotherapy, and sensitivity to radiation can be potentiated by
increasing tumor oxygenation. Efaproxiral, an allosteric modifier, binds
noncovalently to hemoglobin, decreasing its oxygen-binding affinity and
thus increasing oxygenation in tissues. Efaproxiral was studied in a
large phase 3 trial and failed to demonstrate a statistically
significant survival benefit. Subset analysis of breast cancer patients
with brain metastases did show a significant increase in survival and
quality of life, but a confirmatory trial did not replicate the results.
[45] Chemotherapy
Temozolomide Temozolomide, an oral alkylating agent, confers a survival advantage when administered with radiotherapy for glioblastoma.
[46] The drug penetrates the blood-brain barrier (BBB), and when used alone,
produces modest responses in recurrent and newly diagnosed brain
metastases.
[47] Several trials have investigated the efficacy of concurrent temozolomide and WBRT in patients with brain metastases.
[48,49] For example, Antonadou, Verger, and colleagues
[49,50] randomly assigned patients to WBRT alone vs WBRT plus concurrent and
adjuvant temozolomide. The combined therapy demonstrated significantly
higher response rates than did WBRT alone in both phase 2 and phase 3
trials, but no survival benefit was seen in the phase 3 trial.
Polifeprosan 20 With Carmustine Implant Polifeprosan 20 with carmustine implant, a
form of interstitial chemotherapy, contains a biodegradeable polymer
with carmustine (BCNU) and allows chemotherapy to be introduced directly
into the resection cavity following surgery. Brem and colleagues
[51] reported on 42 patients with brain metastases treated with resection
followed by BCNU wafers and WBRT. Mean survival was 16.8 months, and
local control was 100%. By study end, 9 patients (22%) were still alive
with no evidence of central nervous system disease.
Future Developments Brain metastases are a major source of
morbidity and mortality in cancer patients; an ideal future strategy
would focus on preventing brain metastases in the first place. This
approach is currently in use in high-risk populations and is achieved
through prophylactic cranial irradiation, which has been shown to reduce
the risk for relapse and to prolong survival in patients with small
cell lung cancer. It has also been shown to reduce the risk for brain
metastases in patients with NSCLC, but there was no effect on survival
and an increased risk for decline in HVLT-R at early time points.
[52-54] Current clinical trials are focused on decreasing the neurocognitive
risk from cranial radiation; if successful, theseapproaches could permit
greater application of prophylactic cranial irradiation.
In specific patient subpopulations, such as those with HER2/
neu-expressing
brain metastases in breast cancer and EGFR-mutant metastases in NSCLC,
targeted agents, such as trastuzumab, lapatinib, gefitinib, or
erlotinib, could potentially have a role. There is also considerable
interest in evaluating potentially active agents in combination with
WBRT. Despite the BBB penetration limit of trastuzumab, a recent report
showed that when women with HER2-overexpressing breast cancer developed
brain metastases, treatment with WBRT and concomitant trastuzumab
resulted in a > 70% response rate, nearly a 10-month median
progression-free survival, and almost an 18-month median survival.
[55] These results are far superior to what would be expected and support
the contention that when brain metastases develop, some drug probably
permeates through the leaky BBB and potentially acts as a
radiosensitizer. This strategy would be worth testing in a future
randomized trial.
Newer approaches are focused on developing
drugs that cross the BBB and presumably would lower the risk for brain
metastases if used early enough. For example, vardenafil, a
phosphodiesterase inhibitor, was found in mouse models to increase the
amount of trastuzumab that reached brain metastases 2-fold.
[56] This strategy could be tested in a clinical trial, especially if combined with WBRT.
Lin and colleagues
[57] evaluated the safety and efficacy of lapatinib in patients with
HER2-overexpressing brain metastases previously treated with trastuzumab
for metastatic breast cancer and WBRT for brain metastases. Only 1 of
39 patients achieved a partial response in the brain according to the
Response Evaluation Criteria In Solid Tumors, which was below the
hypothesized level of activity (20%), and the median time to progression
was 3.0 months. A subsequent multicenter trial also noted a low 6%
response rate. Although disappointing, these results do not preclude the
possibility of evaluating lapatinib with WBRT or even trastuzumab,
lapatinib, and WBRT.
Another novel approach uses an engineered
peptide compound that targets the low-density lipoprotein
receptor-related protein that is highly expressed on the surface of the
BBB and is upregulated in several tumors. In a phase 1 trial, 48
patients with brain metastases were treated with ANG1005, a taxane
derivative created from this peptide compound. Overall disease control,
including responses plus stable disease, was achieved in 71% of
patients, offering another possible avenue for combining WBRT with
sensitizing doses of chemotherapy.
[58] Finally, brain metastases have also been linked with elevated VEGF expression in murine models.
[59] Bevacizumab has shown promise in the treatment of metastatic NSCLC and colorectal cancer when combined with chemotherapy
[60,61]; future trials could investigate the efficacy of bevacizumab in brain metastases, especially in combination with WBRT.
Supported by independent educational grants from EMD Serono, Merck & Co., Inc., and Genentech, Inc.
References
- Platta CS, Khuntia D, Mehta MP, Suh JH. Current
treatment strategies for brain metastasis and complications from
therapeutic techniques: a review of current literature. Am J Clin Oncol.
2010;33:398-407. Abstract
- Sundstrom JT, Minn H, Lertola KK, et al. Prognosis
of patients treated for intracranial metastases with whole-brain
irradiation. Ann Med. 1998;30:296-299. Abstract
- Gaspar L, Scott C, Rotman M, et al. Recursive
partitioning analysis (RPA) of prognostic factors in three Radiation
Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat
Oncol Biol Phys. 1997;37:745-751. Abstract
- Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W.
A new prognostic index and comparison to three other indices for
patients with brain metastases: an analysis of 1,960 patients in the
RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510-514. Abstract
- Mikkelsen T, Paleologos NA, Robinson PD, et al. The
role of prophylactic anticonvulsants in the management of brain
metastases: a systematic review and evidence-based clinical practice
guideline. J Neurooncol. 2010;96:97-102. Abstract
- Ryken TC, McDermott M, Robinson PD, et al. The role
of steroids in the management of brain metastases: a systematic review
and evidence-based clinical practice guideline. J Neurooncol.
2010;96:103-114. Abstract
- Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7:682-689. Abstract
- Harwood RA, Simson WJ. Radiation therapy of cerebral
metastases: a randomized prospective clinical trial. Int J Radiat Oncol
Biol Phys. 1977;2:1091-1094. Abstract
- Borgelt B, Gelber R, Kramer S, et al. The palliation
of brain metastases: final results of the first two studies by the
Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys.
1980;6:1-9. Abstract
- Al Shamy G, Swaya R. Management of brain metastases: the indispensible role of surgery. J Neurooncol. 2009;92:275-282. Abstract
- Kienast Y, Winkler F. Therapy and prophylaxis of brain metastases. 2010;10:1763-1777.
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized
trial of surgery in the treatment of single metastases to the brain. N
Engl J Med. 1990;322:494-500. Abstract
- Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al.
Treatment of single brain metastasis: radiotherapy alone or combined
with neurosurgery? Ann Neurol. 1993;33:583-590. Abstract
- Mintz AH, Kestle J, Rathbone MP, et al. A randomized
trial to assess the efficacy of surgery in addition to radiotherapy in
patients with a single cerebral metastasis. Cancer. 1996;78:1470-1476.
Abstract
- Bindal RK, Sawaya R, Leavens ME, et al. Surgical treatment of multiple brain metastases. J Neurosurg. 1993;79:210-216. Abstract
- Wen PY, Loeffler JS. Management of brain metastases. Oncology. 1999;13:941-954, 957-961.
- Alexander E 3rd, Moriarty TM, Davis RB, et al.
Stereotactic radiosurgery for the definitive, non-invasive treatment of
brain metastases. J Natl Cancer Inst. 1995;87:34-40. Abstract
- Flickinger JC, Kondziolka D, Lunsford LD, et al. A
multi-institutional experience with stereotactic radiosurgery for
solitary brain metastasis. Int J Radiat Oncol Biol Phys.
1994;28:797-802. Abstract
- Alexander E 3rd, Moriarty TM, Loeffler JS. Radiosurgery for metastases. J Neurooncol. 1996;27:279-285. Abstract
- Shaw E, Scott C, Souhami L, et al. Single dose
radiosurgery treatment of recurrent previously irradiated primary brain
tumors and brain metastases: final report of RTOG protocol 90-05. Int J
Radiat Oncol Biol Phys. 2000; 47:291-298. Abstract
- Andrews DW, Scott CB, Sperduto PW, et al. Whole
brain radiation therapy with or without stereotactic radiosurgery boost
for patients with one to three brain metastases: phase III results of
the RTOG 9508 randomized trial. Lancet. 2004;363:1665-1672. Abstract
- Bhatnagar AK, Flickinger JC, Kondziolka D, et al.
Stereotactic radiosurgery for four or more intracranial metastases. Int J
Radiat Oncol Biol Phys. 2006;64:898-903. Abstract
- Linskey ME, Andrews DW, Asher AL, et al. The role of
stereotactic radiosurgery in the management of newly diagnosed brain
metastases: a systematic review and evidence-based clinical practice
guideline. J Neurooncol. 2010;96:45-70. Abstract
- Rades D, Kueter JD, Veninga T, Gliemroth J, Schild
SE. Whole brain radiotherapy plus stereotactic radiosurgery (WBRT+SRS)
versus surgery plus whole brain radiotherapy (OP+WBRT) for 1-3 brain
metastases: results of a matched pairs analysis. Eur J Cancer.
2009;45:400-404.
- Mueller RP, Soffietti R, Abaciouglu MU, et al.
Adjuvant whole-brain radiotherapy versus observation after radiosurgery
or surgical resection of 1-3 cerebral metastases: results of the EORTC
22952-26001 study. J Clin Oncol. 2009;27:15s. Abstract 2008.
- Patchell RA, Tibbs PA, Regine WF, et al.
Postoperative radiotherapy in the treatment of single metastases to the
brain: a randomized trial. JAMA. 1998;280:1485-1489. Abstract
- Aoyama H, Shirato H, Tago M, et al. Stereotactic
radiosurgery plus whole-brain radiation therapy vs stereotactic
radiosurgery alone for treatment of brain metastases: a randomized
control trial. JAMA. 2006;295:2483-2491. Abstract
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition
in patients with brain metastases treated with radiosurgery or
radiosurgery plus whole-brain irradiation: a randomized controlled
trial. Lancet Oncol. 2009;10:1037-1044. Abstract
- DeAngelis LM, Delattre JY, Posner JB.
Radiation-induced dementia in patients cured of brain metastases.
Neurology. 1989;39:789-796. Abstract
- Regine WF, Huhn JL, Patchell RA, et al. Risk of
symptomatic brain tumor recurrence and neurologic deficit after
radiosurgery alone in patients with newly diagnosed brain metastases:
results and implications. Int J Radiat Oncol Biol Phys. 2002;52:333-338.
Abstract
- ClinicalTrials.gov. Stereotactic radiation therapy
with or without whole-brain radiation therapy in treating patients with
brain metastases. Available at: http://www.clinicaltrial.gov/ct2/show/NCT00377156 Accessed February 24, 2011.
- Schulder M, Black PM, Shrieve DC, Alexander E 3rd,
Loeffler JS. Permanent low-activity iodine-125 implants for cerebral
metastases. J Neurooncol. 1997;33:213-221. Abstract
- Ostertag CB, Kreth FW. Interstitial iodine-125 radiosurgery for cerebral metastases. Br J Neurosurg. 1995;9:593-603. Abstract
- Gallina P, Francescon P, Cavedon C, et al.
Stereotactic interstitial radiosurgery with a miniature X-ray device in
the minimally invasive treatment of selected tumors in the thalamus
and the basal ganglia. Stereotact Funct Neurosurg. 2002;79:202- 213. Abstract
- Chan TA, Weingart JD, Parisi M, et al. Treatment of
recurrent glioblastoma multiforme with GliaSite brachytherapy. Int J
Radiat Oncol Biol Phys. 2005;62:1133-1139. Abstract
- Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive
function and progression in patients with brain metastases treated with
whole brain radiation and motexafin gadolinium: results of a
randomized phase III trial. J Clin Oncol. 2004;22:157-165. Abstract
- Meyers CA, Weitzner MA, Valentine AD, et al.
Methylphenidate therapy improves cognition, mood, and function of brain
tumor patients. J Clin Oncol. 1998;16:2522-2527. Abstract
- Rapp SR, Rosdhal R, D’Agostino RB, et al. Improving
cognitive functioning in brain irradiated patients: a phase II trial of
an acetylcholinestaerase inhibitor (donepezil). Neuro Oncol.
2004;6:357. Abstract QL-09.
- Pellegrini JW, Lipton SA. Delayed administration of
memantine prevents N-methyl-D-aspartate receptor-mediated neurotoxicity.
Ann Neurol. 1993;33:403-407. Abstract
- Peissner W, Kocher M, Treuer H, et al. Ionizing
radiation-induced apoptosis of proliferating stem cells in the dentate
gyrus of the adult rat hippocampus. Brain Res Mol Brain Res,
1999;71:61-68.
- Jaradat H, Khuntia D, Johnson S, et al. Whole-brain
radiation treatment with hippocampal avoidance with tomotherapy. Neuro
Oncol. 2006;8:487. Abstract RO-16.
- Khuntia D, Mehta M. Motexafin gadolinium: a clinical
review of a novel radioenhancer for brain tumors. Expert Rev Anticancer
Ther. 2004;4:981-989. Abstract
- Mehta MP, Rodrigus P, Terhaard CH, et al. Survival
and neurologic outcomes in a randomized trial of motexafin gadolinium
and whole-brain radiation therapy in brain metastases. J Clin Oncol.
2003;21:2529-2536. Abstract
- Mehta MP, Gervais R, Chabot P, et al. Motexafin
gadolinium (MGd) combined with prompt whole brain radiation therapy (RT)
prolongs time to neurologic progression in non-small cell lung cancer
(NSCLC) patients with brain metastases: Results of a phase III trial.
. J Clin Oncol. 2006;24(18S):7014.
- Scott C, Suh J, Stea B, Nabid A, Hackman J. Improved
survival, quality of life, and quality-adjusted survival in breast
cancer patients treated with efaproxiral (Efaproxyn) plus whole-brain
radiation therapy for brain metastases. Am J Clin Oncol.
2007;30:580-587. Abstract
- Stupp R, Mason WP, van den Bent MJ, et al.
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 2005;352:987-996. Abstract
- Abrey LE, Olsen JD, Raizer JJ, et al. A phase II
temozolomide for patients with recurrent or progressive brain
metastases. J Neurooncol. 2001;53:259-265. Abstract
- Antonadou D, Paraskevaidis M, Sarris G, et al. Phase
II randomized trial of temozolomide and concurrent radiotherapy in
patients with brain metastases. J Clin Oncol. 2002;20:3644-3650. Abstract
- Verger E, Gil M, Yaya R, et al. Temozolomide and
concomitant whole brain radiotherapy in patients with brain metastases: a
phase II randomized trial. Int J Radiat Oncol Biol Phys.
2005;61:185-191. Abstract
- Antonadou D, Coliarakis N, Paraskevaidis M et al. A
multiinstitutional trial comparing survival of patients with brain
metastases from lung cancer treated with Temozolomide plus radiotherapy
versus radiotherapy alone. Lung Cancer. 2003;41(suppl 2):42. Abstract
O-67.
- Brem S, Staller A, Wotoczek-Obadia M. Interstitial
chemotherapy for local control of CNS metastases. Neuro Oncol.
2004;6:370. Abstract TA-06.
- Auperin A, Arriagada R, Pignon JP, et al.
Prophylactic cranial irradiation for patients with small-cell lung
cancer in complete remission. Prophylactic Cranial Irradiation
Overview Collaborative Group. N Engl J Med. 1999;341:476-484. Abstract
- Slotman B, Faivre-Finn C, Kramer G, et al.
Prophylactic cranial irradiation in extensive small-cell lung cancer. N
Engl J Med. 2007;357:664-672. Abstract
- Gore EM, Bae K, Wong S, et al. A phase III
comparison of prophylactic cranial irradiation versus observation in
patients with locally advanced non-small cell lung cancer: initial
analysis of Radiation Therapy Oncology Group 0214. J Clin Oncol.
2009;27:383s. Abstract 7506.
- Chargari C, Idrissi HR, Pierga JY, et al.
Preliminary results of whole brain radiotherapy with concurrent
trastuzumab for treatment of brain metastases in breast cancer
patients. Int J Radiat Oncol Biol Phys. 2010 Oct 5. [Epub ahead of
print]
- Hu J, Ljubimova JY, Inoue S, et al.
Phosphodiesterase type 5 inhibitors increase Herceptin transport and
treatment efficacy in mouse brain tumor models. PLoS One.
2010;5:e10108.
- Lin N, Carey LA, Liu MC, et al. Phase II trial of
lapatinib for brain metastases in patients with human epidermal growth
factor receptor 2-positive breast cancer. J Clin Oncol.
2008;26:1993-1999. Abstract
- Sarantopoulos J, Gabrail NY, Moulder SL, et al.
ANG1005: results of a phase I study in patients with advanced solid
tumors and brain metastases. J Clin Oncol. 2010;28:15s. Abstract 2556.
- Yano S, Shinohara H, Herbst RS, et al. Expression of
vascular endothelial growth factor is necessary but not sufficient for
production and growth of brain metastasis. Cancer Res.
2000;60:4959-4967. Abstract
- Hurwitz H, Fehrenbacher L, Novotny W, et al.
Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic
colorectal cancer. N Engl J Med. 2004;350:2335-2342. Abstract
- Socinski MA, Langer CJ, Huang JE, et al. Safety of
bevacizumab in patients with non-small-cell lung cancer and brain
metastases. J Clin Oncol. 2009;27:5255-5261. Abstract



